Introduction
Many Nevada gardeners were “transplanted” here from other areas of the country. When they start gardening in Nevada, they use their tried and true practices from other parts of the country, especially when it comes to amending soil. While many common soil amendments are beneficial when added to soil in the southeastern or northeastern United States or the Pacific Northwest, they are not appropriate for soils in the arid West. They can be detrimental to our soils and water bodies and are, at the very least, a waste of time, effort and money. Two common soil amendments used in other parts of the country are gypsum and lime.
Myth #1: Gypsum is a required soil addition.
Gypsum, or calcium sulfate (CaSO4∙2H2O), is water-soluble, readily breaking down into calcium and sulfate ions. This makes gypsum a good source of plant‐available calcium and sulfur. Sulfur is an essential element in plant protein synthesis. Calcium aids in holding clay particles together, forming crumbles or clods. This improves soil structure by increasing pore space, which in turn improves plant growth and soil biology.
Reality
In Nevada, our soils are already high in calcium and are generally adequate in sulfur.
Physically tight soils, which are often clay‐rich, will not allow water to infiltrate. They can be improved by adding organic material. Even a physically tight soil with a high salt content will benefit more from the addition of organic material than from the addition of gypsum.
The addition of gypsum can help to improve soil structure if a soil has been tested and found to be sodium‐affected. Sodium‐affected soils are not uncommon in our area, but a soil test is required to diagnose this problem. In a sodium‐affected soil, the excessive sodium causes the soil to crust. The sodium ions surround the soil particles, interfering with the formation of crumbles needed for pore space. Adding gypsum to these soils results in a swapping of ions, where the calcium replaces the sodium and the sodium washes away. The calcium ions aid in soil particle crumble formation, creating pore space which improves water infiltration and soil health.
To be effective, the gypsum must be worked into the soil. The soil must then be irrigated sufficiently to dissolve the gypsum and flush away the sodium. The water applied must be low in sodium and the soil must drain adequately so that the sodium can be washed away. Unfortunately, poor drainage and high‐sodium irrigation water are often the main causes of many sodium‐affected soils. You may have to improve drainage and find a low‐sodium water source for irrigation.
Some gardeners feel that gypsum is a great source of sulfur. They add it hoping to improve Nevada’s alkaline soils by lowering the pH. The sulfate ion in gypsum does not decrease soil pH or reduce soil alkalinity. Elemental sulfur worked into the soil will reduce pH, but it must be added to the soil in large quantities so that the effect persists over many years. Changing soil pH is a slow process better achieved by the addition of organic matter than by adding “acidifying” agents.
Plant nutrient problems in alkaline soils
- Iron, manganese and zinc deficiencies
- Phosphorus binds with calcium and magnesium, making it unavailable to plants
Plant nutrient problems in acidic soils
- Aluminum and manganese toxicity
- Calcium, molybdenum and magnesium deficiencies
- Phosphorus tied up with iron and aluminum
- Reduced nitrogen availability
In the event that a sulfur deficiency is suspected, it can be diagnosed with a soil test. Sulfur deficiency symptoms are very similar to those for nitrogen deficiency, with plants showing yellowing on both older and younger leaves and stunted, spindly, thin stems. If testing indicates that a soil is low in sulfur, there are amendments that can be used to increase available sulfur. Many fertilizers, such as ammonium sulfate and sulfur‐coated urea, contain sulfur. Sulfur is sometimes listed as the fourth number on the bag of a complete fertilizer (see sidebar).
Myth #2: Liming should be done annually
Another practice common to gardeners new to Nevada is liming or adding calcium carbonate, calcium‐magnesium carbonate (limestone and dolomite, respectively) or wood ashes to the soil.
Reality
This is a time‐honored and effective method for increasing the pH in very acidic soils. In the arid West, we usually have high pH, alkaline soils rather than acidic soils. We rarely need to increase the pH of a soil or the calcium, potassium or magnesium levels in a soil. Most of our soils have ample free calcium (see sidebar). We generally need to reduce or lower the pH of our soils. This is best accomplished by adding organic matter. Have your soil tested before wasting time and money on soil amendments or fertilizers.
Wondering if your soil has adequate calcium?
Most soils in Nevada have ample calcium in the form of calcium carbonate. This is often called “free lime.” A simple way to test for the presence of calcium carbonate is to take about a tablespoon of your soil, add a teaspoon of vinegar and hold the soil up to your ear. If you hear a fizzing sound, the acid in the vinegar is reacting with the calcium carbonate in the soil, releasing CO2 gas. If you don’t hear a fizzing sound, it doesn’t mean there is no calcium in your soil. The calcium carbonate content may be very low or the calcium may be present in another form. A soil test is the best way to determine your soil’s calcium content.
Calcium carbonate makes it difficult to change soil pH. Acid‐containing substances that enter the soil, such as rainwater, are neutralized by the calcium carbonate in alkaline soils.
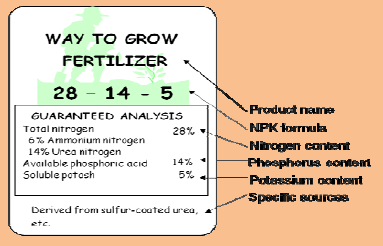
A complete fertilizer is one that contains nitrogen, phosphorus and potassium. These three essential elements are required in the largest quantities for plant growth. The three numbers on the fertilizer bag represent the percentage of these elements in the fertilizer. The first number is percent nitrogen (N), the second is percent phosphorus (as P2O5), and the third is percent potassium (as K2O). This is often referred to as the “N‐P‐K” content.
In a 100‐pound bag of the fertilizer shown above, there are 28 pounds of N, 14 pounds of P2O5 and 5 pounds of K2O. If there is a fourth number, it represents the percentage of sulfur in the fertilizer. For example, a 100‐pound bag of 16‐20‐0‐15 fertilizer contains 16 pounds of nitrogen, 20 pounds of phosphorus, no potassium and 15 pounds of sulfur, with the remaining 49 pounds of material in the bag consisting of inert ingredients. Other nutrients are indicated in parentheses after the N‐P‐K‐S number, such as 0‐0‐21‐ 22 (11 Mg) or in the complete analysis “fine print” on the bag. This formula contains no nitrogen, no phosphorus, 21 percent potassium, 22 percent sulfur and 11 percent magnesium.
References
Donaldson, S., et al, 2008, Living on the Land: Stewardship for Small Acreages, Module 2: Your Living Soil, University of Nevada Cooperative Extension CM‐08‐ 07.
Sawyer, J.E., 2003, Gypsum: An Old Product with a New Use, Integrated Crop Management, Iowa State University Extension IC‐490.
Schalau, J., 2004, Gypsum: Myth vs. Reality, Backyard Gardener, Arizona Cooperative Extension, Yavapai County.
United States Department of Agriculture, Natural Resources Conservation Service, 1999, Liming to Improve Soil Quality in Acid Soils, Soil Quality Institute Technical Note No. 8.
Hefner, M. and Skelly, J.
2009,
Nevada Soil Amendment Myths,
Extension | University of Nevada, Reno, FS-09-15


