Osmosis & Reverse Osmosis (R/O)
Osmosis is the natural tendency of two fluids, separated by a membrane, to equalize salt concentrations. This is the mechanism that plants use to extract water from surrounding soils. It is also how water passes from the intestine into the human body. Osmosis depends on having a membrane that is semi-permeable, allowing some substances, such as water molecules, to pass through while blocking others. Blocked substances include chemicals that are dissolved in water. When two mixtures of chemicals are separated by a membrane that is permeable to water, but doesn't allow chemicals to pass through, water flows from the side that has the lowest concentration of chemicals to the side that has the highest concentration (Figure 1).
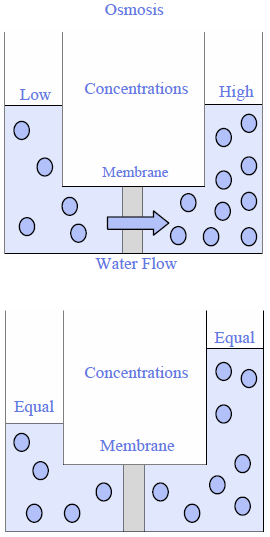
Figure 1: Osmosis
To illustrate this concept imagine a leather bota bag filled with salt water in a swimming pool filled with fresh water. Leather is a semi-permeable membrane. Oils pass through to lubricate the skin, but other fluids are retained. Water will slowly flow from the swimming pool into the bag to try to equalize the concentrations of dissolved solids on both sides of the leather. As this occurs the water pressure will rise in the leather bag, to the point where the bag bursts or flow stops because the difference in pressure between the two sides of the membrane can no longer drive flow into the bag. The pressure caused by differences in chemical concentrations is called osmotic pressure. The strength of pressure depends on the difference in concentration of chemicals on either side of a semipermeable membrane.
Reverse osmosis relies on a semipermeable membrane and pressure to remove chemicals dissolved in water. Using the example of the leather bag, if a few bricks are placed on top of the bag, the pressure inside increases until it reaches the osmotic pressure created by difference in chemical concentrations. Because of the pressure, fresh water will flow from the bag through the leather leaving the salts inside the bag.
This is essentially what happens with reverse osmosis units that are available for home use. High concentrations of chemicals remain trapped inside a permeable membrane that has pores small enough to allow water to pass through (Figure 2). Chemicals are trapped inside the membrane and flushed away as wastewater. Pressure comes from the water pressure that is part of normal operation of household water supplies—whether from a well or from a public water supply system that pipes water to a home. Reverse osmosis represents a reverse of normal osmotic processes because pressure has been applied on the side of the membrane where chemical concentrations are highest (Figure 3).
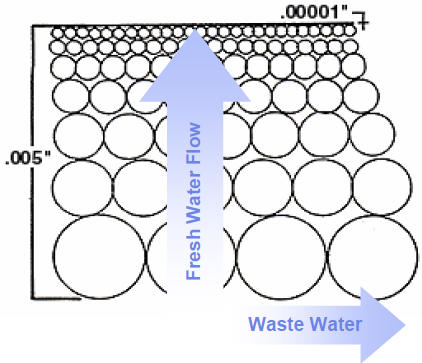
Figure 2: An example of a membrane cross-section The flow rate of water through the membrane is controlled
by the membrane thickness, size of the pores, and the difference in pressure across the membrane (1)
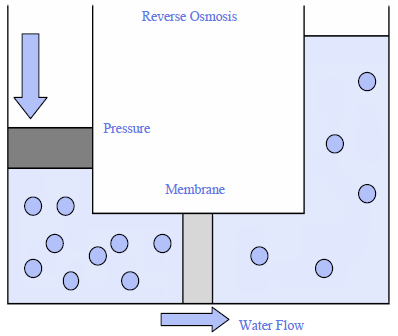
Figure 3: Reverse Osmosis
History of Commercial R/O
The original commercial reverse osmosis systems were designed for use on ships to produce fresh water during long voyages. These systems used high-pressure pumps to force water through membranes. The first membranes used for reverse osmosis were very thick, which made treatment systems quite large. These were suitable for industrial processes and large-scale treatment, but not for home use. Early reverse osmosis equipment was so large that even a moderate-sized unit would not fit under a typical kitchen sink.
In the early stages of commercial development of reverse osmosis several vexing problems were encountered. One of the more problematical ones was how to attach the membranes to a backing material without using adhesives that would coat the membrane and render it useless. In some cases the membranes fell off of the backing as soon as pressure was reduced. Under these circumstances the membrane tore when flow was restored in the unit.
A second difficulty encountered was the size of the membrane tubes themselves. The amount of freshwater produced is proportional to the surface area of the membrane and the pressure applied. One way to increase the rate of treatment is to increase the pressure on the membrane and hope that it does not tear or leak. The other is to increase surface area. This can be done by using many small tubes instead of one large tube as a membrane holder.
Both of these problems were solved with improved membranes, adhesives and the use of plastics. The results led to products that can be applied for home use, with pressures that can be expected from normal plumbing in households.

Original commercial R/O systems were designed for use on ships to produce fresh water during long voyages
Applying a R/O System for Water Treatment
A basic reverse osmosis system consists of a pressurized source of water, a membrane system, and hoses or pipes to carry off and separate clean water and wastewater. Most systems also include a prefiltration system that consists of a carbon filter and a cartridge filter, a pressure tank to store the clean water, and a stainless steel or plastic faucet to dispense the clean water (Figures 4 & 5). The cartridge filter is necessary for residences that are connected to a private well, to trap sand and dirt particles. The carbon filter removes chlorine, hydrogen sulfide, organic chemicals (such as solvents and fuels) and other objectionable materials that may be in the water supply but will not be effectively removed by a reverse osmosis system.

Figure 4: An example of a R/O system (2)
Several factors can be varied to speed up the production of clean water. Membrane design is very important. The flow rate through the membrane is controlled by membrane thickness, size of the pores, and the difference in pressure across the membrane. There are practical limits to the efficiencies that can be obtained by changing these. Although flow rates increase as the pressure increases, high pressure may tear the membrane. Tearing creates holes that allow chemicals to pass through.
Membrane thickness can also be varied. The thinner the membrane, the faster clean water will be produced by the system. However thin membranes are more susceptible to tearing and damage. Most modern membrane systems are constructed of an extremely thin semi-permeable polymer film bonded to a support backing. Thin films are difficult to produce without defects that allow contaminants to pass through the membrane. Thicker membranes are more durable and easier to produce. This is similar to painting a wall with latex paint. Ideally it is preferable to use the thinnest coat of paint possible to make a gallon of paint last long. However the thinner the layer of paint, the more likely it is that there will be spots on the wall where the old color shows through. Similarly, thin spots in a membrane may allow water to flow through without removing chemicals.

Figure 5: An example of a R/O faucet used to dispense clean water (3)
How Much Water is Wasted by a R/O System?
Reverse osmosis units are usually used to produce small quantities of water. Reverse osmosis taps are often installed next to the main faucet (Figure 5). Reverse osmosis systems discharge wastewater to prevent build-up of contaminants. The ideal volume of the water discharge is based upon several factors, including the design of the unit, type and amount of contaminants in the feed water and water temperature. When high concentrations of contaminants are in feed water, more flow is necessary to sweep the membrane surface clean.
Manufacturers test membrane systems at a standard temperature of 77 degrees Fahrenheit. For every degree less than this, flow rates across the membrane decrease by 1 to 2 percent. In other words if the feed water temperature is 60 degrees, the unit will produce water at a rate of about 15% to 25% less than rated and produce about 15% to 20% more waste water. When choosing a water treatment system it is important to determine how much water is needed to produce one gallon of treated water. In the case of most reverse osmosis water treatment systems operating near 77 degrees Fahrenheit, it takes two to four gallons of water to produce one gallon of treated water. This can be an important concern if, for example, the septic system or well are near capacity prior to the installation of a reverse osmosis unit. The addition of a reverse osmosis system could also noticeably raise water bills in areas with metered water.
The higher the concentration of contaminants present in feed water, the more flow is necessary to sweep the membrane surface clean.
The designs of these systems vary and some of them are much more water efficient than others. Some of the more expensive systems incorporate a switch that will shut off feed water when a water storage tank is full. Some of the inexpensive systems do not incorporate this feature and as a result water is constantly flowing through the unit, even when the storage tank is full.
When water is treated using reverse osmosis the portion of the water that does not pass through the membrane contains a higher concentration of minerals than it did prior to its entering the system. There is a limit to the quantity of a material that will dissolve in water. This is known as solubility. If the solubility of a mineral is exceeded in the membrane system after the clean water is extracted, it will drop out of the water or precipitate and could clog the system. Table 1 shows some substances that can lead to clogging. It is a good idea to test water before you purchase a reverse osmosis system, to determine if the concentration of some chemicals could lead to clogging.
Table 1: Under some conditions, chemical concentrations may lead to clogging and other problems with R/O units
| Chemical |
Conditions |
| Alkalinity |
If alkalinity exceeds 1000 ppm and the majority of the alkalinity is due to carbonate rather than bicarbonate, materials could precipitate |
| Barium |
If barium concentrations exceed 1.5 ppm, barite (barium sulfate) precipitate may form |
| Boron |
Water containing more than 50 ppm boron and an alkalinity of greater than 1000 ppm may form precipitates |
| Calcium and Magnesium |
If the pH of the membrane water is greater than 10 and the combined calcium and magnesium content exceeds 60 ppm, precipitates may form |
| Hardness |
Hardness is a measure of the combined total concentration of calcium, magnesium, and strontium in water. If pH exceeds 10, alkalinity exceeds 500 ppm, and hardness exceeds 150 ppm, precipitates may form |
| Iron and Manganese |
Combined concentrations of iron and manganese greater than 25 ppm can lead to formation of precipitates |
| Silica |
The concentration of silica should be less than 100 ppm |
| Sulfate |
If water has a pH greater than 10 and contains sulfate greater than 50 ppm, it may produce a precipitate |
| Turbidity |
For proper operation the turbidity of feed water should be less than 1 NTU |
Additional Extension Publications
- Water Testing for Private Well Owners (SP-00-02)
- Matching Drinking Water Quality Problems to Treatment Methods (SP-00-19)
- Drinking Water Quality in Nevada (FS-00-46)
- Reverse Osmosis (R/O): Installing and Maintaining a Reverse Osmosis Unit (FS-05-10)
- Reverse Osmosis (R/O): Selecting a Reverse Osmosis Unit (FS-05-09)
Photo Credits
- Jim supplies
- APEC
- Crystel Montecinos
This publication was produced with support from
The Nevada Agricultural Experiment Station at the University of Nevada, the Drinking Water Revolving Loan Fund administered by the Nevada Division of Environmental Protection and a Regional Water Quality Coordination Grant provided by the U.S. Department of Agriculture’s Cooperative State Research, Extension and Education Service.
Fisher, A., Reisig, J., Powell, P., and Walker, M.
2005,
Reverse Osmosis (R/O): How It Works,
Extension | University of Nevada, Reno, FS-05-08


