Have Water Tested First
Before deciding upon any treatment system for home drinking water, be sure to obtain a thorough chemical analysis. Some chemicals can lead to clogging that reduces fresh water production, as explained in “Reverse Osmosis (R/O): How it Works” (FS-05-08). If a municipal water system or a water company serves your home, they can provide a copy of the results of tests that they are required to perform regularly. If your water comes from a private well, have the water analyzed by a laboratory certified by the state for drinking water analysis. No single water treatment system is designed to treat all water problems. Under the right circumstances, such as for water that contains high concentrations of dissolved minerals, a reverse osmosis unit is an excellent choice.
Installation of Reverse Osmosis (R/O) Systems
Typically the total system will take up between one third and one half of the space beneath a sink. The largest component of a reverse osmosis system is the pressure tank that holds treated water and supplies a separate faucet. In general, this tank is about half the size and about the same shape as propane tanks used for outdoor barbeque grills. It is important that the tank be placed in an upright position to prevent leakage. It must be kept in a heated area to prevent freezing in the winter.
The reverse osmosis unit itself is generally one to two feet long and is meant to be hung from a vertical surface beneath the sink. It, like the pressure tank, must be protected from freezing, which will destroy the reverse osmosis membrane. During installation keep in mind that it will be necessary to access the unit for maintenance from time to time. Its location on the wall or the side of the cabinet should allow enough room to change filter cartridges.
Reverse osmosis units are for use on the cold water system only. Reverse osmosis units are not recommended for installation in households served by wells that produce hot water (> 77° F) from geothermal sources. Hot water will destroy the membrane, which is designed for cold-water use only.
Most home units produce small volumes (a gallon of clean water every two to four hours) so treated water is available in limited quantities. This means that under the sink reverse osmosis units will not be a good choice for any activity that requires large amounts of water, such as washing clothes, showering, or bathing.
Common Chemicals Removed by R/O Systems
Reverse osmosis was originally designed to produce potable water from seawater and brackish sources. It can be applied in most cases to remove dissolved inorganic materials (minerals). It is also a good technology to remove some pesticides. A re-verse osmosis unit readily removes asbestos, because asbestos fibers are too large to pass through the membrane. Table 1 lists substances that can be removed or reduced by a reverse osmosis unit. The National Sanitation Foundation maintains a web site that identifies specific brands of reverse osmosis systems and tests system efficiency in removing chemicals (NSF).
R/o is Not Suitable for All Water Problems
There are several things that a reverse osmosis system is less than effective in removing. First and foremost, sand and silt scratch and quickly destroy the membrane. A reverse osmosis unit is not ideal for removing bacteria, petroleum, or other materials that form a slime. Slimy materials will coat the membrane, preventing water from passing through it.
Dissolved gases and materials that readily turn into gases also can easily pass through most reverse osmosis membranes. This means that reverse osmosis will be ineffective for wells contaminated with natural gas and chloroform. High levels of chlorine, such as those typical of shock chlorination of wells, can damage the membrane and seriously affect treatment efficiency. Many reverse osmosis units have an activated carbon unit to remove or reduce the concentration of most organic compounds, and much of the chlorine.
A chemical called hydrogen sulfide causes a “rotten egg smell.” It is naturally produced when organic material decomposes in the absence of oxygen and is also often found in wells with dissolved natural gas. The form that the hydrogen sulfide takes in the water is dependent upon pH. If the pH is less than six, the hydrogen sulfide will be a gas and cannot be removed by a reverse osmosis unit. If the pH is greater than eight in water that is being treated (feed water), the hydrogen sulfide will occur as sulfide ions that can be removed. A more cost effective way to remove hydrogen sulfide is to use activated carbon. Activated carbon is available in many forms, from cartridge filtration systems to stand alone units that resemble a water softener.
Membrane Efficency
Reverse osmosis systems, while being an effective method to remove many substances from water, do not remove all chemicals. Table 2 lists some of the materials that reverse osmosis membranes are not ideally suited to remove. In order to determine how efficiently a reverse osmosis membrane system is operating a standard test solution has been developed to test the removal rate of some common ions. The percentage of the ions removed at a standard temperature and pressure is referred to as the membrane efficiency. The actual results obtained at home may vary due to differences in water chemistry, pH, water temperature, and numerous other factors. Membrane efficiency estimates are useful to compare different reverse osmosis systems but do not guarantee how well the system will operate under any unique set of conditions. Efficiency depends on water chemistry and maintenance.
Table 1: Chemicals That Can Be Removed or Reduced by a R/O Unit
| Chemical |
Comment |
| Aluminium |
< 5 ppm. Higher levels will plug membranes |
| Antimony |
Effectively removed at commonly found concentrations |
| Arsenic |
Removal rate is better for pentavalent arsenic than trivalent arsenic |
| Bicarbonate |
Effectively removed at commonly found concentrations |
| Bismuth |
Effectively removed at commonly found concentrations |
| Boron |
Effectively removed as borate |
| Calcium |
Low levels are readily removed, high levels form membrane scale |
| Chromium |
Effectively removed at commonly found concentrations |
| Cobalt |
Effectively removed at commonly found concentrations |
| Copper |
Effectively removed at commonly found concentrations |
| Cyanide |
Removes cyanide ion only in alkaline solutions |
| Fluoride |
Effectively removed as fluoride ion |
| Iron |
< 5 ppm. |
| Lead |
Effectively removed at commonly found concentrations |
| Lindane |
Effectively removed as the pure form of the pesticide |
| Manganese |
Effectively removed at commonly found concentrations |
| Magnesium |
Removed in waters with low to moderate hardness |
| Mercury |
Effectively removed as mercuric or mercurous ion |
| Molybdenum |
Effectively removed as molybdate ion |
| Nickel |
Effectively removed at commonly found concentrations |
| Nitrate |
Effectively removed at commonly found concentrations |
| PCB |
Effectively removed at commonly found concentrations |
| Perchlorate |
Effectively removed at levels < 100 ppm |
| Pesticides |
Some may not be removable; check with R/O manufacturer |
| Radium |
Effectively removed at commonly found concentrations |
| Selenium |
Effectively removed as selenate and selenite. |
| Silica |
Effective at levels < 50 ppm |
| Silver |
Effectively removed at commonly found concentrations |
| Sodium |
Effectively removed at commonly found concentrations |
| Strontium |
If carbonate levels are excessive, this may clog the membrane |
| Sulfate |
Effectively removed at commonly found concentrations |
| Tellurium |
Effectively removed at commonly found concentrations |
| Tin |
Effectively removed at commonly found concentrations |
| Tungsten |
Effectively removed at commonly found concentrations |
| Uranium |
Effectively removed at commonly found concentrations |
| Vanadium |
Effectively removed at commonly found concentrations |
| Zinc |
Effectively removed at commonly found concentrations |
Table 2: Materials R/O Membranes Do Not Remove Well
| Chemical |
Comment |
| Aluminum |
Concentrations in feed water greater than 5 ppm can lead to membrane clogging |
| Bacteria |
R/O units will remove bacteria but are not recommended for this use |
| Benzene |
Not effective |
| Calcium |
High levels of calcium can result in membrane clogging |
| Chloroform |
Not effective |
| Chlorine |
Low levels of chlorine pass through the membrane. High levels destroy the membrane |
| Cyanide |
Does not remove cyanide under acidic conditions or most organocyanides (nitriles) |
| Iron |
Concentrations greater than 5 ppm can result in membrane clogging. |
| Jet Fuel |
Standard household membranes are not designed to remove so |
| Magnesium |
Under high hardness conditions, magnesium carbonate scale may clog membranes |
| Mercury |
Does not remove methyl mercury |
| Naphthalene |
Not effective |
| Pesticides |
Some are not removed |
| Radon |
Home R/O systems are not designed to remove gasses |
| Sand |
Will destroy membranes |
| Selenium |
R/O units do not remove hydrogen selenide |
| Silica |
Silica at levels above 50 ppm can precipitate in the membrane cartridge and clog the system |
| Sulfide |
Not removed at pH of less than 7 because it occurs as hydrogen sulfide gas. Rate of removal increases with increasing pH |
| Toluene |
Not effective |
| Tritium |
Not effective |
Relative Costs of Bottled Water and R/O System Water
The costs of using reverse osmosis to produce drinking water are an important consideration. In fact, some may wonder if bottled water would not be a solution to home water quality problems. To compare costs of using bottled water, a homeowner should know the cost of buying and installing a reverse osmosis system, the estimated useful life of the system, the costs of regular maintenance (including replacement parts), and the expected volume of water consumed per day. The volume is most easily compared in terms of fluid ounces, because most bottled water is provided in bottles that contain about one pint (16 fluid ounces) (Note: 1 gallon = 128 fl oz). As an example, suppose a reverse osmosis system costs $300 to buy and install and that the estimated useful life of the system is four years. Also assume that replacement parts and maintenance cost $80 per year and that the homeowner wants to produce two gallons of clean water per day. Over the four year life of the system in 2005 the homeowner would want:
This would cost:

To estimate the cost per fluid ounce, divide the total number of fluid ounces needed by the total cost. In this example, the cost per fluid ounce would be $620÷373,760 fl oz— approximately $0.002 or 2/10 of a penny. Note that this simple analysis does not consider other factors such as the costs associated with buying and storing bottled water. Most supermarkets provide cost per unit figures to help shoppers compare prices. By carrying out the simple analysis described above, a homeowner can evaluate the relative costs of bottled water and reverse osmosis water to compare the costs of producing water and buying bottled water.
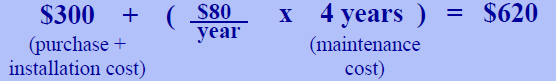
R/O is Best for Limited Use
There are R/O systems that can treat the entire water supply for the home instead of just what is used at one faucet. It should be noted that these systems are extremely expensive. For the most part other far more cost-effective systems can be used to improve water quality for the entire house. Also, water treated by a reverse osmosis system that does not have a system to add some dissolved minerals back into the system (a rehardener) is corrosive to copper pipes and brass faucets. Operation of a whole house system without a rehardener can result in severe corrosion of copper water lines and may add lead and copper into drinking water.
R/O and Foul Smelling Hot Water
Two conditions cause hot water to smell bad. Most commonly, the chemical that causes the offensive odor is in the incoming water. Examples of this include petroleum-like odors. These are more noticeable in hot water than cold water, because the chemical becomes a gas at higher temperatures. The more gas present, the stronger the odor that is perceived. Organic-type odors such as petroleum and rotten egg are most cost effectively removed from the whole house system by using an activated carbon filtration system.
The odor may also be due to biological growths in the hot water tank. The most expedient method to rid a shower of these odors is to use a one-time shock chlorination process to disinfect the supply well. If this is done, make sure that enough hot water is run out of the tap to ensure that an adequate amount of chlorine has reached the water heater to kill the offending biological growth.
Water Pressure and the R/O System
The standardized water test for reverse osmosis uses 60 PSI water pressure. The actual water pressure is not as important as the pressure that forces water through the membrane. The more contaminants present in feed water, the higher the pressure needed to force clean water through the membrane. However, there is a limit to how much pressure the membrane will tolerate before cracking. The limits vary between units and are specified in instructions that are part of the packaging. DO NOT EXCEED THE MANUFACTURER’S RECOMMENDATIONS ABOUT WATER PRESSURE.
Before Buying
Compare the estimated costs of installing and operating a reverse osmosis system with the cost of buying bottled water. It may be that the convenience of the reverse osmosis system costs more than using bottled water. Test your water to be sure that the temperature and chemical conditions are right for efficient operation. Compare prices and brands. The website for the National Sanitation Foundation (NSF) provides useful information to help compare and select reverse osmosis units.
Additional Resources
- Water Testing for Private Well Owners (SP-00-02)
- Matching Drinking Water Quality Problems to Treatment Methods (SP-00-19)
- Drinking Water Quality in Nevada (FS-00-46)
- Reverse Osmosis (R/O): Installing and Maintaining a Reverse Osmosis Unit (FS-05-10)
- Reverse Osmosis (R/O): How It Works (FS-05-08)


